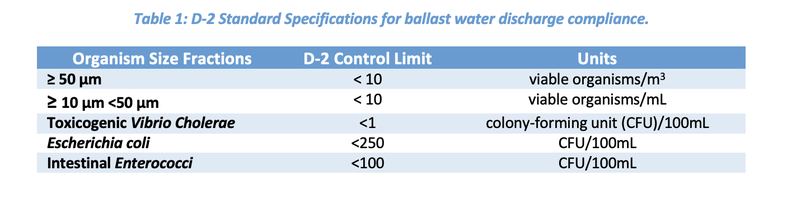
Overview
Ballast tanks within ships are filled with water to provide stability during transportation of cargo. The ballast water is drawn up from the ships departure location (uptake), and later released at its destination port (discharged). The ballast water that is discharged at the ship’s destination may carry invasive species or pathogens that can disrupt the ecology of delicate marine environments and have substantial environmental and economic impacts. For this reason, the International Maritime Organization (IMO) introduced a Ballast Water Management Convention treaty to implement standards and compliance for ballast water management. The IMO standard D-2 outlines the maximum number of viable organisms allowed to be discharged (Table 1) per volume of ballast water in each of the sample fractions based on the size of organisms. This standard will need to be met by all ships by 2024, hence the need for ballast water management systems (BWMS) to decrease biomass to compliant levels.
Adenosine triphosphate (ATP) is present in all living cells acting as an energy transporter in metabolic processes. Since it is found in all living cells, it can be
used as a true indicator of living organisms in a sample. The LuminUltra BQUA PLUS Ballast Water Monitoring Kit is specialized for measurement of ATP in ballast water to provide clear indications
of discharge compliance in the three organism size fractions in the D-2 Standard. The BQUA PLUS kit is accurate, rapid, and portable, allowing it to be used on board vessels or in a
laboratory.
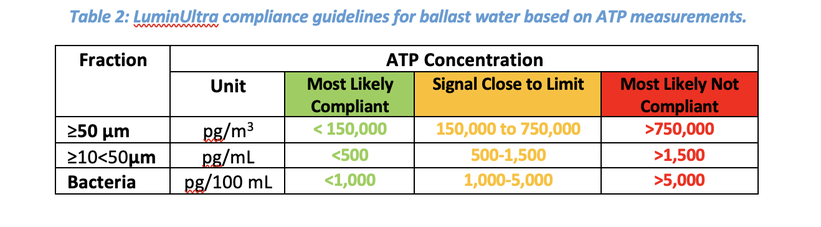
In this study, the BQUA PLUS kit was assessed by Ankron Water Services GmbH, a marine testing and consulting company. It was tested during a commissioning testing study for samples with simulated BWMS discharge water using chlorine and UV treatment. An indicative analysis using a compliance monitoring device (CMD) is required to validate the commissioning of a BWMS as per IMO regulation BWM.2/Circ.70/Rev.1. The commissioning test is successful if the discharge samples are compliant with the D-2 Standard according to the indicative analysis method used, which are listed in BWM.2/Circ.42/Rev.2. If the samples are non-compliant after treatment, a detailed analysis must be performed to confirm the results. The corresponding ATP concentration guidelines for the D-2 control limits are specified in Table 2.
Methodology
I. Samples
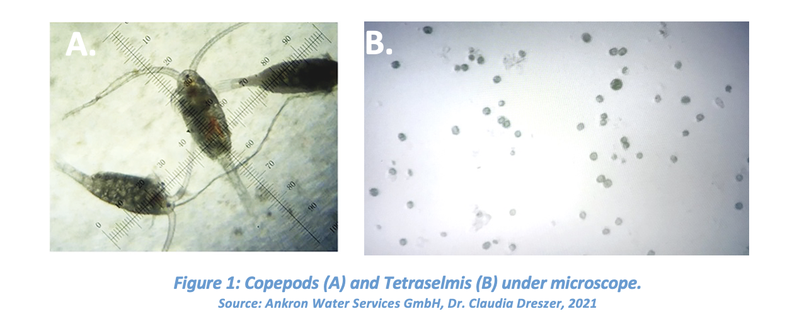
The samples used in this study were artificial marine water spiked above the D-2 discharge limits with organisms that correspond to each size fraction so the BWMS efficacy can be assessed. Copepods (zooplankton) were added to the artificial marine water to make up the ≥50 µm fraction, and Tetraselmis (phytoplankton) were used for the ≥10 < 50 µm fraction (Figure 1). The phytoplankton was added to the ≥10 < 50 µm fraction to 104 cells/mL. For both BWMSs being tested, an untreated artificial uptake sample was compared to treated artificial discharge samples.
II. Analysis Methods
The analysis methods used to quantify organisms in the ≥10 < 50 µm fraction included two detailed (MPN, FDA/CMFDA) and two indicative (PAM, ATP) methods:
· Most Probably Number (MPN): MPN is a growth-based method that determines the viable cell count. MPN will not capture cells that are viable but not culturable since these cells will not grow in the nutrient medium.
· Fluorescein diacetate (FDA)/5-Chloromehtylfluorescein diacetate (CMFDA): The FDA/CMFDA dyes react with enzymes associated with substrate metabolism to emit fluorescence. The sample can then be placed under a fluorescent microscope and the stained cells can be counted. Cells with damaged DNA (non-viable) may be labelled as live cells using this method since their enzymes may still be active.
· Pulse Amplitude Modulation (PAM): PAM detects photosynthetic microorganisms by measuring chlorophyll concentration and can give an insight on the biological activity of phytoplankton.
· ATP measurements: The BQUA PLUS kit uses the luciferase enzyme isolated from fireflies which emits light upon reacting with ATP. The light is then measured in a luminometer to quantify ATP in the sample.
To quantify the ≥50 µm fraction, one detailed (microscopy) and one indicative (ATP) method was applied.
· Microscopy: direct count of organisms that show any signs of movement or activity.
· ATP measurements: Application of the BQUA PLUS kit.
III. Ballast Water Management Systems
Under the chlorine BWMS, the spiked artificial marine water was subject to a chlorine dosage of 8 ppm with a 24-hour holding time. A UV BWMS was simulated using a Collimated Beam Device, where the spiked artificial marine water was subjected to a UV dose of 1.200 J/m2 before and after a 24-hour holding time for a total UV dose of 2400 J/m2. Both the chlorine and the UV dosages used in this study are standard for ballast water management using these systems.
Results and Discussion
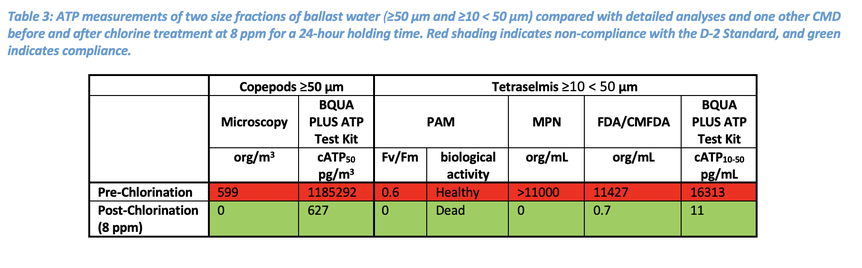
The BQUA PLUS ATP data for the chlorine BWMS agreed with the microscopy quantification for the ≥50 µm fraction, reading well below the D-2 compliance threshold of 150,000 pg/m3 for this fraction after chlorine treatment (Table 3). Similarly, the ATP data for the ≥10 < 50 µm fraction agreed with PAM, MPN and FDA/CMFDA methods in measuring a compliant number of organisms after treatment compared to the pre-chlorinated uptake. The commissioning testing of the chlorine BWTS was validated for both the ≥50 µm and ≥10 < 50 µm size fractions by the BQUA PLUS ATP test kit and agreed with the other CMD and detailed analyses.
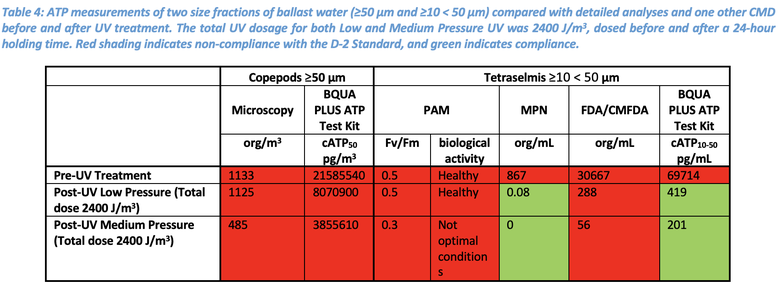
The BQUA PLUS ATP data for the ≥50 µm fraction of the UV treatment agreed with the microscopy results indicating non-compliance (Table 4). In the ≥10 < 50 µm fraction, the ATP data agreed with the MPN results in showing compliance post-UV treatment compared to the pre-UV treated uptake sample. The PAM and FDA/CMFDA results, however, indicated non-compliance. The PAM and FDA/CMFDA results are likely false positives due to their detection of chlorophyll and metabolic products which can both remain in cells after being rendered non-viable by the UV treatment. The MPN and BQUA PLUS ATP methods were not impacted due to their detection of viable cells only and reported compliance after UV treatment.
conclusion
Compared with other indicative compliance monitoring tools, the BQUA PLUS test kit that measures ATP concentration of live cells is one of the only tests capable of measuring all three of the D-2 standard size fractions of ballast water: ≥50 µm, ≥10 < 50 µm, and <10 µm (bacteria). This study demonstrates the suitability of the BQUA PLUS kit as an indicative analysis tool for commissioning testing of ballast water management systems, particularly in its accurate assessment of the ≥10 < 50 µm fraction after UV treatment. In addition to its ability to accurately detect live cells, the BQUA PLUS ATP assay gives conclusive and rapid results for indicative testing, allowing Ankron Water Services GmbH and other companies performing commissioning testing on BWM systems and avoiding the cost and time needed for excessive detailed analyses.
Contact Ankron Water Services: Dr. Claudia Dreszer, claudia.dreszer@ankron.de
